@charset "utf-8";
/* CSS Document */
Live events — Global events, expert speakers and webinar talks
Meet us here
×

We are attending INTA 2024 Atlanta!
Meet our IP experts at booth #827, May 18-22, 2024.
Sign up
Innovation transforms
SO DO WE

The AI patent analysis platform to enhance your innovation
Spotlight Octimine: Our patent search and analysis platform has a brand-new website.
Optimize costs and value of your IP portfolio
Reduce Intellectual Property costs smartly and extract maximum value from your intangible asset portfolio.
-
-
-
Previous
Next
Patent deep search and monitoring tools
Automated patent analysis and monitoring tools based on Deep Learning improve sharpness and quality of patent searches. Octimine also features patent landscaping, regular checks help you staying on top of trends and budget for your Intellectual Property assets.
Explore Octimine Search
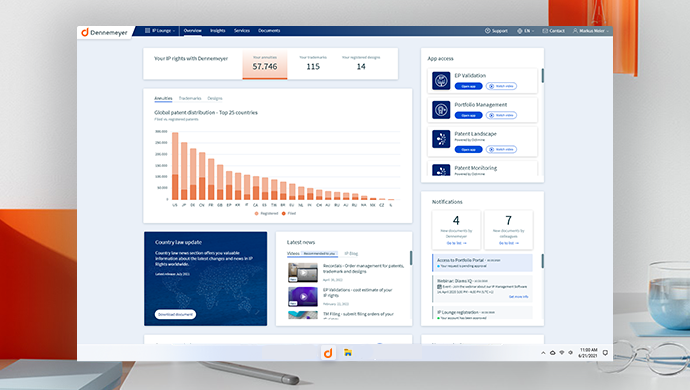
Convenient central access to all apps
Register your customer account
IP Lounge customer center simplifies the access to all booked digital tools and apps such as Portfolio Management, EP Validation, TM Filing, and other advanced IP management systems. Check personalized dashboard graphs, news, and status, manage user data or perform searches. Are you already a Dennemeyer client? Welcome to the IP Lounge!
The IP Group
Global Intellectual Property management
Combining expertise in local IP procedures with the scope of a worldwide full-service provider, the Dennemeyer Group is your Intellectual Property management partner wherever you do business.
Contact sales
Full service for IP portfolios
In addition to the full spectrum of IP-related legal services, Dennemeyer offers IP strategy consulting, IP management software, IP payment services and cutting-edge patent search and analysis tools.
In addition to the full spectrum of IP-related legal services, Dennemeyer offers IP strategy consulting, IP management software, IP payment services and cutting-edge patent search and analysis tools.
Truly worldwide coverage
With 20+ offices across the globe, Dennemeyer manages over three million IP rights for around 8,000 customers. We are committed to securing IP rights everywhere our clients operate.
With 20+ offices across the globe, Dennemeyer manages over three million IP rights for around 8,000 customers. We are committed to securing IP rights everywhere our clients operate.
Regional IP expertise
Managing IP rights on a global scale requires exhaustive knowledge of local markets and IP regulations. Dennemeyer’s team of Intellectual Property specialists has the insights into regional specifications.
Managing IP rights on a global scale requires exhaustive knowledge of local markets and IP regulations. Dennemeyer’s team of Intellectual Property specialists has the insights into regional specifications.
Single point of contact
We offer a convenient and friendly interface to any IP issue with a dedicated contact in your language. Whenever you need help or advice for IP assets, we are there to support.
We offer a convenient and friendly interface to any IP issue with a dedicated contact in your language. Whenever you need help or advice for IP assets, we are there to support.
Scalable IP solutions
Intellectual Property solutions respond to the size of your business and how this can change over time. Strategically expand and retract the IP services you require from us. Your ambition is never static, and neither are we.
Intellectual Property solutions respond to the size of your business and how this can change over time. Strategically expand and retract the IP services you require from us. Your ambition is never static, and neither are we.
Advanced IP technology
Software, applications, and platforms make the latest methods available. We simplify your workday with digital IP services that can learn, that are intelligent and intuitive. Professionalism and customer focus meets state-of-the-art legal tech and processes.
Software, applications, and platforms make the latest methods available. We simplify your workday with digital IP services that can learn, that are intelligent and intuitive. Professionalism and customer focus meets state-of-the-art legal tech and processes.
Managing, maintaining and updating large numbers of IP assets on a global scale
We manage all actions and services related to ownership data for IP rights. Whether you have transferred, divided or acquired assets, we ensure that all records of ownership and address are accurate and up to date.